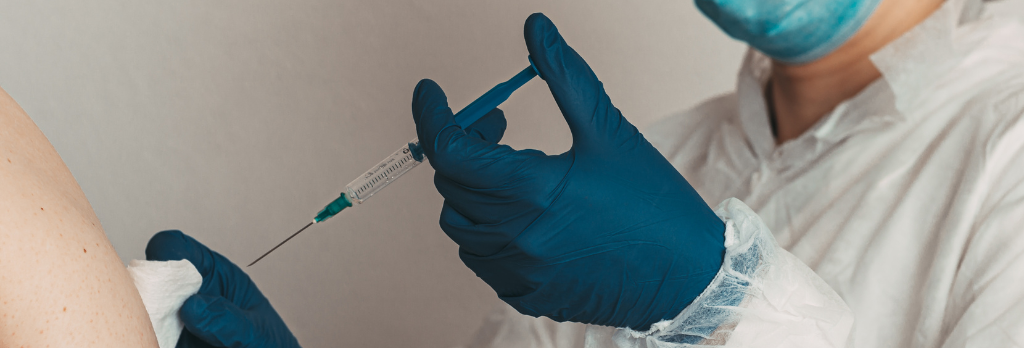
What to Expect at Your Appointment to Get Vaccinated for COVID-19
September 26, 2022Everyone 6 Months and Older Should Get a COVID-19 Vaccine
COVID-19 vaccination has many benefits and is an important tool to help protect you from severe illness, hospitalization, and death.
Even if you or your child have had COVID-19, you should still get yourself or your child vaccinated.
- Getting a COVID-19 vaccine after having COVID-19 provides added protection against the virus that causes COVID-19.
- People who already had COVID-19 and do not get vaccinated after their recovery are more likely to get COVID-19 again than those who get vaccinated after their recovery.
- If you were given monoclonal antibodies or convalescent plasma while sick with COVID-19 you do not need to wait to get vaccinated.
Before the Vaccination
If you do not regularly take over-the-counter medications, you should not take them before you get a COVID-19 vaccination.
It is not known how over-the-counter (OTC) medicines, such as ibuprofen, aspirin, or acetaminophen, might affect how well the vaccine works. You may be able to take these types of medications to reduce fever or pain after you get your vaccine to relieve any pain or discomfort resulting from possible side effects.
Get a COVID-19 vaccine with your routine medical procedures and screenings
You can combine most procedures, screenings, and vaccinations at the same appointment when you get your COVID-19 vaccination. Talk to your healthcare provider if you have questions.
Children, teens, and adults may get a COVID-19 vaccine and other vaccines, including a flu vaccine, at the same time.
Preparing children and teens for vaccination
If you are getting your child or teen vaccinated learn how you can support them and talk to them about what to expect. The experience of getting a COVID-19 vaccine will be very similar to that of getting routine vaccines.
Requesting accommodations at COVID-19 vaccination sites
- When making an appointment or arriving for vaccination, you can let staff and/or volunteers know you or your child might need some accommodations.
- People with disabilities can use the COVID-19 Vaccine Disability Information and Access Line (DIAL) to get help with COVID-19 vaccinations.
If you have allergies related to vaccines
Talk to your doctor if you:
- have had a severe allergic reaction to a previous dose to learn if you should get a different type of COVID-19 vaccine,
- are allergic to polyethylene glycol (PEG) and you should not get Pfizer-BioNTech or Moderna COVID-19 vaccine,
- are allergic to polysorbate and you should not get Novavax or J&J/Janssen COVID-19 vaccine
- if you are allergic to other types of vaccines or injectable medications for other diseases.
- If you had an immediate allergic reaction (a reaction that started within 4 hours of getting vaccinated) to a COVID-19 vaccine, but the reaction was not considered severe by a medical professional, you can receive another dose of the same vaccine under certain conditions. Your doctor may refer you to an allergy and immunology specialist for more care or advice.
- If you have had an immediate allergic reaction—even if it was not severe—to a vaccine or injectable therapy for another disease, you should discuss this with your doctor to determine which COVID-19 vaccine is best for you.
If you have allergies not related to vaccines
You should get vaccinated if you have allergies that are not related to vaccines or injectable medications such as food, pet, venom, environmental, or latex allergies. People with a history of allergies to medications taken by mouth or a family history of severe allergic reactions can also get vaccinated.
At the Vaccination Site
- You should receive a paper or electronic version of a fact sheet that tells you more about the COVID-19 vaccine you or your child received. Each approved and authorized COVID-19 vaccine has its own fact sheet that contains information to help you understand the risks and benefits of that vaccine.
- There is no charge for your COVID-19 vaccine. Your COVID-19 vaccine is free. COVID-19 vaccines are paid for with taxpayer dollars and are given free of charge to all people living in the United States, regardless of health insurance or immigration status. If anyone asks you to pay for a COVID-19 vaccine, it’s a scam.
After Getting a COVID-19 Vaccine
- Stay on site to be monitored for at least 15 minutes.
- Make sure your vaccination provider updates your vaccination card (or gives you one if this is your first dose).
- Stay up to date with the recommended COVID-19 vaccines and boosters.
- You may experience side effects after getting a COVID-19 vaccine.
- Adverse effects (serious safety problems) and severe allergic reactions are rare.
- To report any side effects, you can sign up for v-safe. V-safe is a smartphone-based tool that provides quick and confidential health check-ins via text messages and web surveys so you can quickly and easily share with CDC how you or your dependent feel after getting a COVID-19 vaccine.
Watch Video: Use v-safe to tell CDC how you’re feeling after COVID-19 vaccination [00:00:34]
Your CDC COVID-19 Vaccination Card
Keep your CDC COVID-19 vaccination card for proof of vaccination. Consider taking a picture of your card after each of your COVID-19 vaccination appointments as a backup copy.
- Bring your card to your appointment whenever you get a primary series dose or booster so that your provider can fill in information about your shot.
- If your vaccine card is full, your vaccine provider can give you another card.
- If you did not receive a CDC COVID-19 vaccination card at your first appointment, contact the vaccination provider site where you got your first shot to find out how you can get a vaccination card. You can also contact your state health department to get a copy of your vaccination record.
- Some vaccination providers and health departments may offer you access to a QR code or digital copy of your COVID-19 vaccination card in addition to giving you a physical CDC COVID-19 vaccination card. Contact your vaccination provider or local health department to learn if a digital copy of your card is available to you.
- If you were vaccinated abroad there are ways you can update your U.S. vaccination record.
- To report suspicious activity involving fake CDC COVID-19 vaccination cards, please visit Fraud Alert: COVID-19 Scams or call 1-800-HHS-TIPS.
To learn more, please visit https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect.html.